14th August
An important component of the ISO 14644 series of cleanroom standards has been updated - ISO 14644-5: 2025. Entitled ‘Cleanrooms and associated controlled environments —Part 5: Operations’, it sets out the basis of an Operational Control Programme (OCP) for cleanrooms. Taking the form of a document impact assessment, it suggests that each facility puts a OCP in place.
With cleanrooms, these are ‘rooms within which the number concentration of airborne particles is controlled and classified, and which is designed, constructed and operated in a manner to control the introduction, generation, and retention of particles inside the room’ (ISO 14644-1: 2025, section 3.1.1).
The revised standard addresses:
This article assesses the primary changes.
Cleanrooms and associated controlled environments assure the control of contamination required for contamination-sensitive activities, including aseptic processing. With this regard, consistent quality depends on cleanliness and specified cleanliness levels for contaminants should be in place. Once established, these quality levels should be attained and maintained through a deliberate programme to establish and implement adequate design and operational procedures.
The ISO 14644 series of standards, to which regulations like EU GMP Annex 1 refer, provide design measures and test requirements to support cleanroom quality objectives.
The ISO 14644-5: 2025 standard specifies the basic requirements for cleanroom operations. It is intended for those who design, construct, start up or operate a cleanroom.
To maintain quality and control, the following sequence of steps is normally observed (Figure 1):
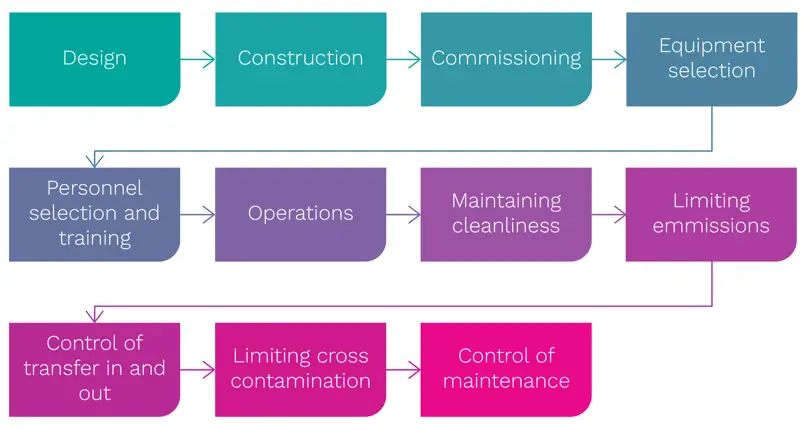
Figure 1: Essential design and operational steps for a cleanroom
To develop the OCP (with the final output being a control strategy document), each of the main operational areas need to be examined to determine what is in place and then to assess if any gaps remain.
A useful approach is:

In terms of identifying gaps and considering a remediation plan, the following table provides a checklist of areas to consider for the exercise:
Table 1: Assessment of operational areas
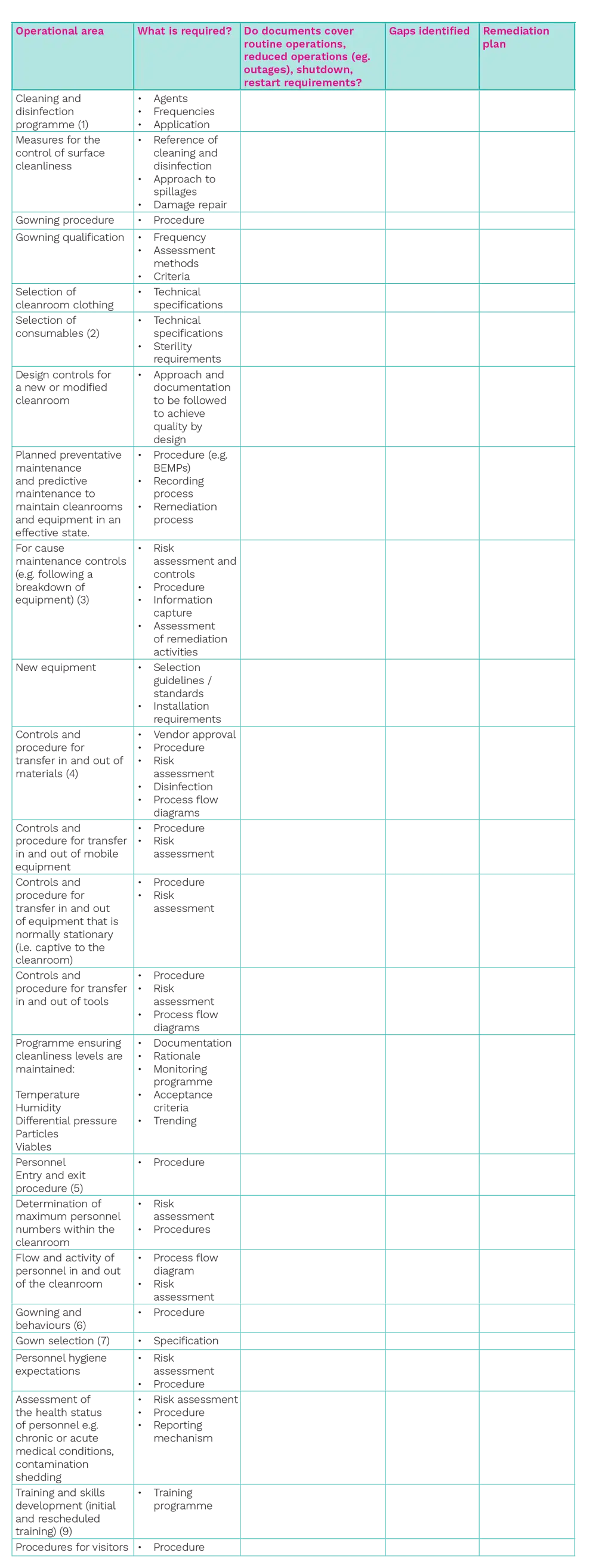
With Table 1:
1. The cleaning and disinfection programme should include frequencies, cleaning instructions, cleaning sequences, detail of the areas and surfaces to be cleaned, preparation steps, methods of application and expiry times. Requirements for special cleaning / disinfection, such as coming out of shutdowns, must be provided. The cleaning programme should take into consideration a range of different surface types.
The standard recommends:
a) Floors, subfloors (underneath a raised floor) and mezzanines
b) Walls, doors, return grilles, windows and other vertical surfaces
c) Ceilings, diffusers, lamp fixtures and other fixtures upstream or close to work areas
d) Stationary equipment
e) Tools (upon entry or when kept inside of the cleanroom)
f) Tables, workstations and other critical horizontal surfaces
g) Cleanroom furniture, such as chairs and ladders
h) Cross-over benches, supply and cleanroom clothing supply cabinets, lockers and other compartmented surfaces
i) Carts and trolleys
j) Rubbish bins and containers
k) Cleanroom mats and sticky flooring
2. Personal consumables, e.g., garments, gloves and face masks must be appropriately selected and justified as per ISO 14644-18.
3. Special partitioning or intensified monitoring may be required. It is strongly recommended for maintenance or repairs to be done when the cleanroom is not in use (welding, soldering and grinding all present a major risk of particles). An assessment should be made of cleaning / disinfection and monitoring requirements.
4. Only materials, equipment and cleaning agents that are evaluated and found acceptable for use in the cleanroom should be permitted in the cleanroom. A formal written procedure should list out all acceptable materials. Protective packaging for transport or storage must be removed outside the cleanroom and the airlock. The materials should be cleaned and disinfected to an agreed particulate level compatible with the room’s specification.
5. Physical or time-based separation between ingoing and outgoing cleanroom personnel should be in place to reduce the impact of cross-contamination. Exiting aseptic processing areas should be based on a one-way flow design. Personnel maps should be in place to show entry and exit routes.
6. Personal items, such as jewellery, that are commonly worn or used outside controlled environments may cause cleanroom or product contamination. They also have the potential to tear or impact the barrier function of gloves and gowning. Jewellery, including piercings on exposed skin, rings, earrings, necklaces, watches, bracelets and pins should not be permitted. Cleanroom personnel should not use or apply a personal product (e.g., lotion, soap, cosmetic, fragrance) unless it has been approved by contamination control.
Cosmetics, including but not limited to foundation, eyeliner, mascara, false eyelashes, lipstick, eyebrow pencil, blusher, nail polish, false tan, temporary tattoos and talcum powder, plus perfumes, are not permitted. Eating, drinking, smoking, vaping and chewing gum are not permitted.
Minimum change out requirements must be established - some people may need to change more often since the frequency of change outs is dependent on individual personnel. Some people will naturally perspire more than others, or some cleanroom activities can dirty the cleanroom garments faster (e.g., foreign object debris generation, contact with the floor).
7. Gown choice includes fabric selection to meet the barrier, strength and lint properties as needed to maintain the required level of cleanliness. Detail of repair and laundry requirements must be provided. Justification of the lifetime of each garment must also be provided.
8. Training should cover:
a) Cleanroom operation procedures
b) Personal hygiene procedures
c) Entry and exit of personnel
d) Gowning requirements and procedures
e) Necessary procedures and work instructions, including cleanroom procedures to maintain cleanliness levels
f) Transfer of materials and equipment. This can include items that are approved for use in cleanrooms and those not approved for use
g) Cleaning procedures where applicable
h) Behaviour in cleanrooms to minimise the generation of contamination that can be transferred or deposited on or into the product
i) Monitoring levels, such as alert and alarm levels and required responses
j) Individual behaviour and responsibilities for cleanroom access and operations
k) Procedures for problem reporting and encouraging cleanroom personnel to report potential issues
l) Requirements for individual personnel certification for cleanroom access and how often recertification is required
m) Personnel monitoring programme
n) Safety
In addition to the above, the OCP should connect with the change control process to enable changes proposed to operational cleanrooms to go through the following assessment:
a) Utilities (compressed gasses, water systems, chemicals)
b) Vibration
c) Electromagnetic fields (e.g., interference, compatibility)
d) Electrostatic properties of materials
e) Lighting (e.g., natural, artificial and non-visible wavelength)
f) Chemical emissions
g) Thermal load from equipment
h) Changed airflow patterns due to equipment placement
i) Modified standard operating procedures
Pulling these components together provides the basis of a holistic cleanroom operations policy.
The primary change to the 2025 edition of ISO 14644-5 is the recommendation for facilities to develop a documented Operations Control Programme (OCP). The OCP connects with the Contamination Control Strategy (CCS) concept and provides the basis for understanding whether the facility cleanrooms and clean air device operates within their specified quality requirements, centred on cleanliness levels. By integrating material and personnel flow diagrams, procedures for operation, maintenance and shutdown, cleaning, disinfection, monitoring programmes and maintenance schedules, a holistic overview of the cleanroom suite is provided.
ISO 14644-1: Cleanrooms and associated controlled environments - Part 1: Classification of air cleanliness by particle concentration, International Standards Organisation, Geneva
ISO 14644-5: Cleanrooms and associated controlled environments - Part 5: Operations, International Standards Organisation, Geneva
ISO 14644-18: Cleanrooms and associated controlled environments - Part 18: Assessment of suitability of consumables, International Standards Organisation, Geneva