The vials were initially triaged; i.e. they were inspected and particles, if present, were located, photographed and measured in situ (Figure 1) without accessing the contents of the vials. Identified particles were grouped based on common optical features. Results of this initial investigation were reported to the client.
The client then reviewed the data and relevant vials were selected for further analysis. Particles were isolated and analysed by compound light microscopy, Fourier Transform Infrared (FT-IR) spectroscopy (Figure 2) and X-ray microanalysis (Figure 4).


Figure 1 - micrograph of the foreign particle in the drug product.
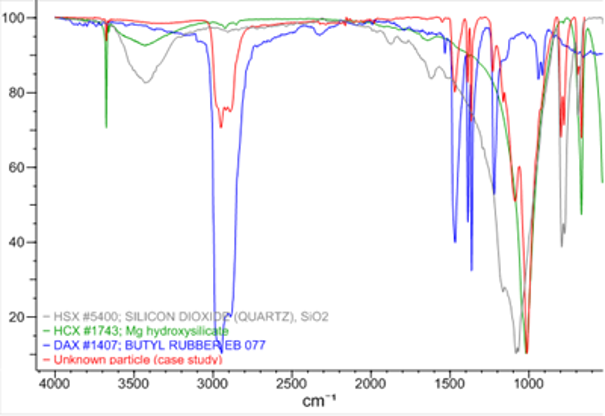
Figure 2 - FT-IR spectrum of the particle (red) compared with reference spectra of butyl rubber (blue) and siliceous material (green and grey).
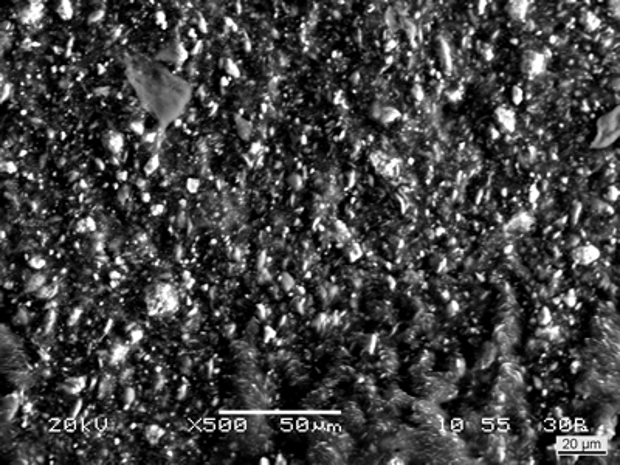
Figure 3 - Backscattered electron image of a subsample of the foreign particle; the ‘bright’ specks corresponded with material (siliceous minerals) of a higher atomic weight.
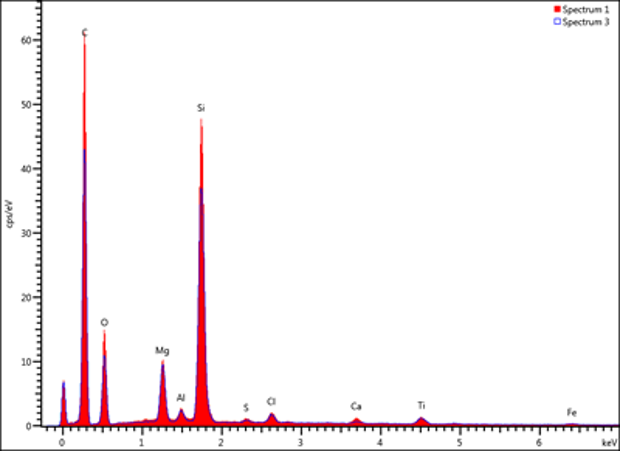
Figure 4 - X-ray microanalysis spectral overlay typical of the particle isolated from the vial (solid red and blue outline).
The data demonstrated that a large number of the particles in the vials had originated from the rubber stoppers. The client was then able to successfully address and resolve this contamination concern by liaising directly with their stopper supplier.