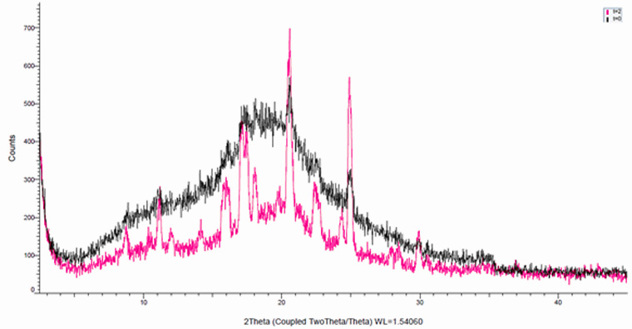
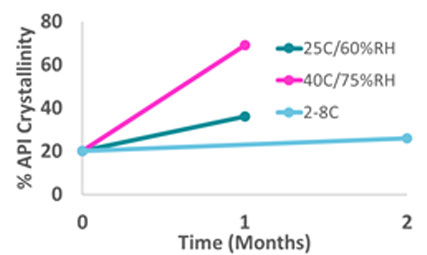
The challenge
Active Pharmaceutical Ingredients (API) that are broken down in the gastrointestinal (GI) tract are not suitable for oral administration and therefore an alternative route needs to be considered. Pulmonary delivery via the lungs is an alternative route, however not all APIs can be manufactured into a respirable formulation. The nasal cavity has been considered as a route for drug administration for many decades, however, this has traditionally only been used for localised treatment of nasal congestion, allergic rhinitis, and infections. The nasal anatomy, physiology and aerodynamics severely limit the potential access to a large mucosal surface that is well suited for drug delivery. Powder intranasal formulations are still very much in their infancy and therefore require a large degree of formulation and device development.