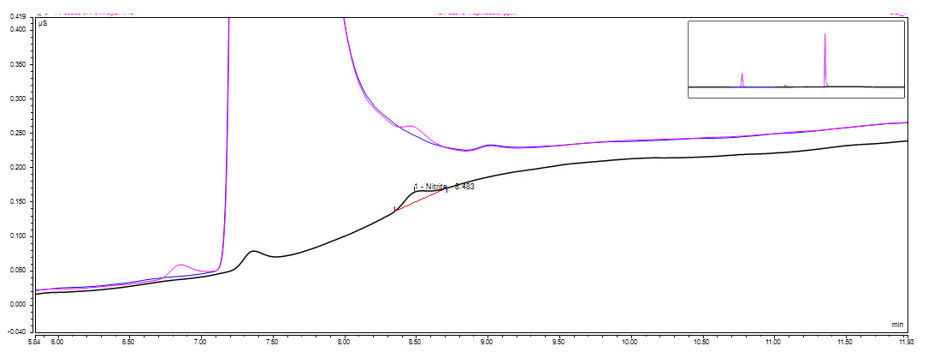
Nitrites and nitrates are commonly found in pharmaceutical products as preservatives or stabilizers. However, the presence of these compounds can lead to the formation of potentially carcinogenic nitrosamines. Nitrosamines are formed when nitrites or nitrates react with amines, which can be present in drug substances, excipients, or contaminants.
The control of nitrosamine formation is essential to ensure the safety and efficacy of pharmaceutical products. Nitrosamines have been identified as significant impurities in some drugs, including ranitidine, which resulted in product recalls and regulatory actions worldwide.
Nitrite and nitrate analysis is important in controlling nitrosamine formation as it helps to monitor the levels of these compounds in pharmaceutical products. By measuring the concentration of nitrites and nitrates in drug substances and finished products, manufacturers can identify potential sources of nitrosamine formation and take appropriate measures to prevent it