BY DR TIM SANDLE | 24 JULY 2023
Catch up with the latest news from around the pharmaceutical industry with issue 11 of our regulatory review, curated by Dr Tim Sandle.
Interested in receiving RSSL's monthly regulatory round-up by email? Email us today to sign up for our pharmaceutical regulatory newsletter
Comments on ICH M11 guideline: Clinical study protocol template and technical specifications - Scientific guideline
A new harmonised guideline is being developed to introduce a clinical protocol template and the technical specification to ensure that protocols are prepared in a consistent fashion and provided in a harmonised data exchange format acceptable to the regulatory authorities.
The ICH M11 Clinical Electronic Structured Harmonised Protocol Template provides comprehensive clinical protocol organisations with standardised content including both required and optional components.
The technical specifications that are acceptable to all regulatory authorities of the ICH regions presents the conformance, cardinality and other technical attributes that enable the interoperable electronic exchange of protocol content with a view to develop an open, non-proprietary standard to enable electronic exchange of clinical protocol information.
Public consultation has been completed within Europe and the comments received are available on the EMA website, here: https://www.ema.europa.eu/en/documents/comments/overview-comments-received-ich-m11-guideline-clinical-study-protocol-template-technical_en.pdf
Blue-box requirements for pharmacies – updated
“Blue–box” requirements concern additional information on labelling/package leaflet that may be required nationally in accordance with Articles 57 and 62 of Directive 2001/83/EC as amended. These requirements apply to products authorised via a national, mutual recognition or decentralised procedure only.
The “blue-box” requirements document has been updated, the 2023 update can be found here: https://www.hma.eu/fileadmin/dateien/Human_Medicines/CMD_h_/procedural_guidance/Application_for_MA/CMDh_258_2012_Rev26_2023_05_clean_-_BlueBox_requirements.pdf
EU antimicrobial resistance activities
The European Commission has drawn up a proposal to strengthen EU action against antimicrobial resistance (AMR). The aim is to help combat AMR in the fields of human, animal and environmental health, following the so-called ‘One Health’ approach.
The recommendation focusses on infection prevention and control, surveillance and monitoring, innovation and availability of efficient antimicrobials, prudent use and cooperation among member states and globally. This includes:
- 20% reduction of the total consumption of antibiotics in humans
- At least 65% of the total consumption of antibiotics in humans should be effective (use of the right antibiotic)
- A reduction of infections of three key antibiotic-resistant bacteria, which will apply mainly to hospitals
See: https://data.consilium.europa.eu/doc/document/ST-9581-2023-INIT/en/pdf
FDA issues draft guidance on tattoo inks
The U.S. FDA has issued draft guidance to tattoo ink manufacturers and distributors to help recognise situations in which a tattoo ink may become contaminated with microorganisms, and thus, be potentially injurious to health. This follows on from a situation in May 2019, when the FDA issued a safety alert advising consumers, tattoo artists and retailers to avoid using or selling certain tattoo inks contaminated with microorganisms. Called out of being of particular concern are pseudomonas aeruginosa and bacillus cereus.
The guidance also recommends certain steps that manufacturers and distributors could take to help prevent the occurrence of these conditions, or to identify and remediate insanitary conditions that already exist during manufacturing and distribution.
The FDA also urges consumers and healthcare providers to report adverse reactions from tattoos.
The draft guidance can be found here: https://www.fda.gov/media/169265/download
Key performance indicators (KPIs) to monitor the European clinical trials environment
The European Medicines Agency has updated the document ‘Key performance indicators (KPIs) to monitor the European clinical trials environment’.
The report provides an overview of Key Performance Indicators (KPIs) related to the implementation of the Clinical Trials Regulation.
This report is published as part of the business change programme Accelerating Clinical Trials EU (ACT EU), involving the European Commission, the Heads of Medicines Agencies (HMA), Clinical Trial Coordination Group (CTCG) and the EMA.
Guideline on the Quality Aspects of mRNA Vaccines
The European Medicines Agency has issued a ‘Concept Paper on the Development of a Guideline on the Quality Aspects of mRNA Vaccines’.
The concept paper addresses the need to establish a guideline on the quality aspects of mRNA vaccines. The number of clinical trial applications for human products and marketing authorisation applications for mRNA containing products has significantly increased over the last few years and is expected to increase further in the future. Furthermore, a lot of experience with mRNA vaccines was gained during the COVID-19 pandemic. From an analytical and regulatory perspective, mRNA vaccines are interesting since their classification depends on the target and/or whether they are obtained chemically or biologically.
mRNA vaccines against infectious diseases have to align with the general guidance for human vaccines, however new technology is not fully accounted for in the existing guidance. It is therefore proposed to establish a guideline addressing those specific aspects regarding the manufacturing process, characterisation, specifications and analytical control as well as the definition of active substance and finished product for mRNA vaccines for the prevention of infectious disease.
The scope of the guideline is limited to mRNA vaccines against infectious diseases (including self-amplifying mRNA). mRNA-based therapeutics will be out of scope of the document. It is not intended to address specific requirements for mRNA vaccines to be used in clinical trials, however the scientific principles described may be applicable during pharmaceutical development.
For details see: https://www.ema.europa.eu/en/documents/scientific-guideline/concept-paper-development-guideline-quality-aspects-mrna-vaccines_en.pdf
Excipients
European Pharmacopoeia (#PhEur) Supplement 11.2 came into force in 39 European countries on 1 July 2023. It contains some updated excipient monographs that have been revised to include a section on functionality related characteristics (such as the physical characteristics and chemical characteristics of an excipient that influence one or more of its functions when used in specific applications in the final product).
For example, with the Polysorbate-80 (0428) monograph (used as an emulsifier or solubiliser in parenteral and non-parenteral liquid dosage forms and in semi-solid preparations) a cross-reference to the tests for composition of fatty acids and hydroxyl value is now included.
Medical devices: legal requirements for specific medical products
The MHRA has updated its guidance for the classification of specific medical products and custom-made prosthetic, orthotic and ophthalmic devices. The guidance is specific to medical devices placed on the market in Great Britain.
The government has extended acceptance of CE marked devices in Great Britain. For more detail on this see the implementation update on work towards a strengthened future medical devices regime.
See: https://www.gov.uk/government/publications/medical-devices-legal-requirements-for-specific-medical-devices/medical-devices-legal-requirements-for-specific-medical-devices
Immunotoxicity
The U.S. FDA has produced a guidance document on the immunotoxic potential of pharmaceuticals. This sets out the requirements for pharmaceutical product development, considering different pharmacological impurities that can supress or stimulate the immune system.
The guidance is titled “Nonclinical Evaluation of the Immunotoxic Potential of Pharmaceuticals”. See: https://www.fda.gov/media/169117/download
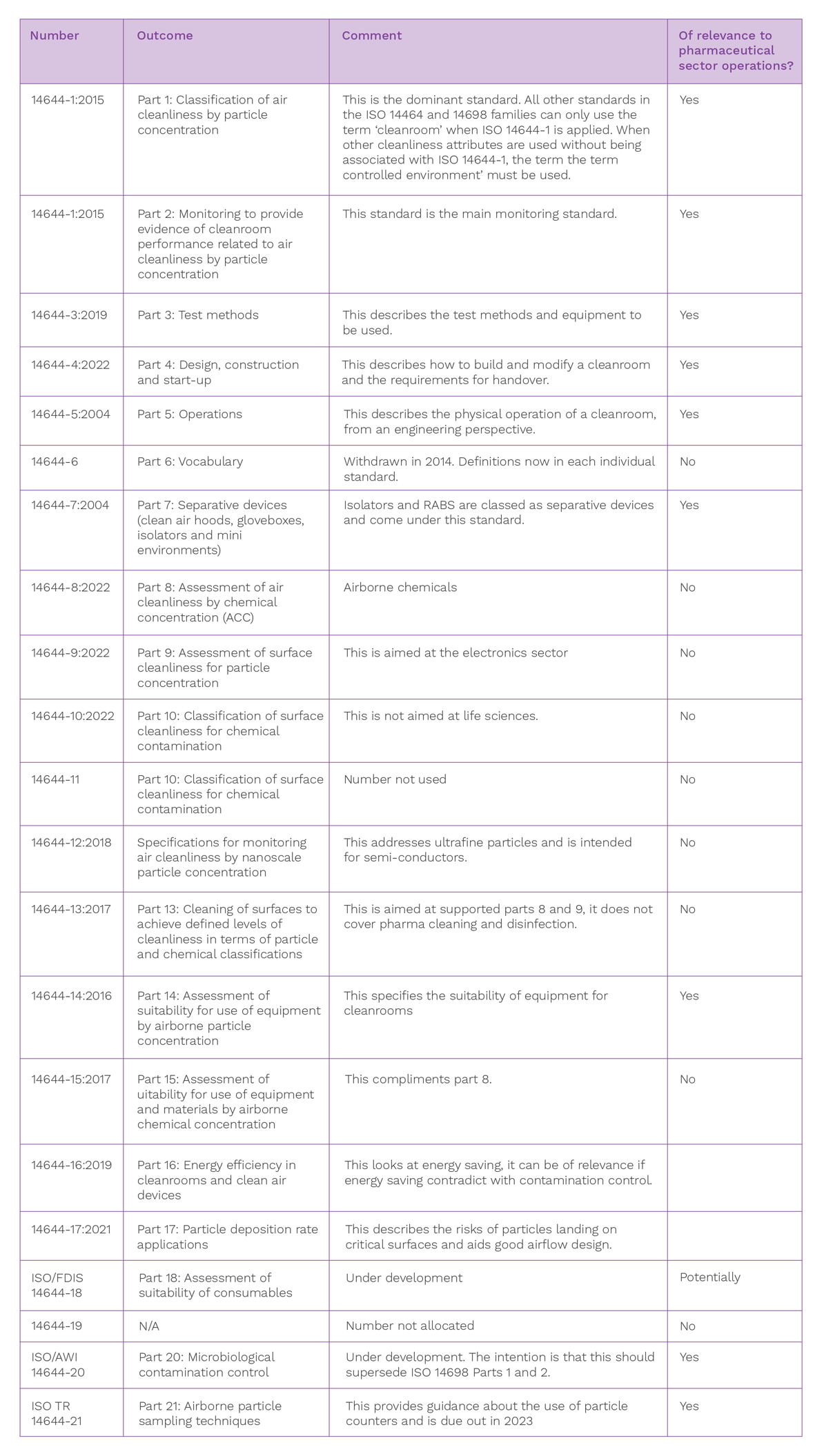
Cleanroom standards
The table below provides an update on the cleanroom standards that form part of the ISO 14644 series.