BY DR TIM SANDLE | 14 MARCH 2023
Interested in this topic? Find out more about designing a cleaning validation strategy with our latest webinar.
Cleaning validation verifies the effectiveness of cleaning processes within pharmaceutical and healthcare facilities. It should be directed to situations or process steps where contamination or the carryover of materials pose the greatest risk to product quality (1), as evaluated to appropriate limits (2). To ensure cleaning process robustness, care must be taken in the selection of cleaning chemicals. This article looks at the choices available and some of the important selection factors for cleaning chemicals.
To determine the most appropriate chemicals, an understanding of the products is needed, especially with the selection of the most appropriate in-process material for the cleaning validation (3). This choice should be based on factors like solubility, difficulty of cleaning, the different types of equipment to be cleaned and the calculation of residue limits based on potency, toxicity, and stability.
Cleaning steps
The primary steps when cleaning an item of equipment include (4):
1. Removal of debris
2. Initial rinsing of residues with water
3. Application of a detergent (ideally with physical action)*
4. Rinsing with water
5. Addition of other chemicals e.g. acids**
6. Rinsing with water
7. Drying
8. Sanitisation or sterilisation (such as, by chemicals or by heat)***. Chemical sanitisers require removal, followed by rinsing and drying. Heat processes include hot water or low pressure steam. For sterilisation, the methods are steam under high pressure or irradiation.
*It is generally the case that detergents are alkali based. Increasing the temperature when using the cleaning solution tends to increase the soil removal efficiency. The heated molecules possess high kinetic energy which serves to dislodge soil faster compared with slow-moving molecules in a cold solution (5).
**The use of acids may be required to remove scaling or alkaline residues. There may be circumstances where the acid can aid the removal of certain microorganisms. Acids, especially hydrochloric acid, can corrode certain metals, therefore care is required. Using a surfactant with the acid will lower its corrosivity, but this will not prevent corrosion. Phosphoric or nitric acid are less corrosive and tend to be preferred.
***Common sanitisation agents are oxidising agents - hypochlorite solutions (potassium, sodium or calcium) peracetic acid, or chlorine dioxide.
Effective cleaning is more likely to be achieved where quality by design principles have been considered, especially with automated clean-in-place systems. Weaknesses with design include surface roughness, clean welding and the presence of inaccessible areas or corners where dirt and/or cleaning chemicals may build up.
Typology of cleaning chemicals
There are a range of different cleaning chemicals available to facilitate cleaning validation. The chemicals can be divided in different ways based on their chemical type and chemical reaction. Agents include detergents (surfactants), which lower the surface tension of residue with the help of surfactant addition in water. Wetting reduces surface tension, improves the rate of dissolution and promotes residue disintegration. The detergent may also contain chemicals that can act against specific residues.
When using detergents, understanding the appropriate concentration is important since over-diluted detergents may not be as effective and could even lead to bacterial tolerance. Conversely, high concentrations of detergent could lead to product adulteration and safety issues. It should also be noted that detergents can be affected by water pH and hardness levels.
In terms of chemical reaction, the two primary forms are: hydrolysis, as used with detergents, and oxidation, which relates to chemical sanitisers.
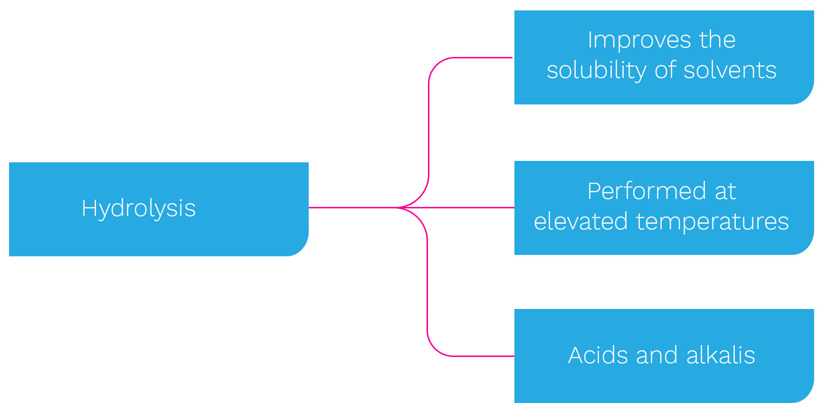
Figure 1: Application of hydrolysis
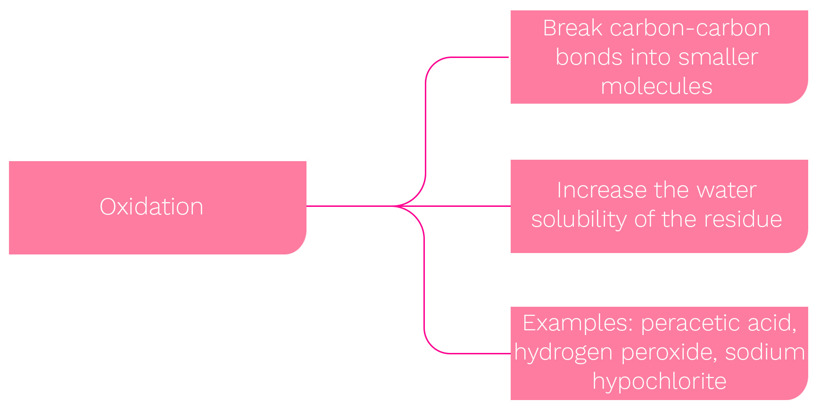
Figure 2: Application of oxidation
While some of the above chemicals have theoretical antimicrobial properties, they should not be seen as disinfecting in the sense that disinfection is defined (i.e., the known reduction of a population of microorganisms as demonstrated through controlled laboratory studies).
Many cleaning processes utilise hydrolysis. There is a choice between alkalis, acids, or a combination of the two. With alkalis, the common chemical is caustic - sodium hydroxide (NaOH). This has a high pH and is commonly used at a concentration range of 0.5 to 4.0%. Sodium hydroxide is especially adept at softening fats and protein. Alkalis possess positively charged ions to promote chemical reactions. With acids, where required, these follow the use of an alkali. Nitric acid, hydrochloric acid, or phosphoric acid are the most commonly used washes. These are generally used for scale removal and pH stabilisation after the caustic wash. The typical concentration is 0.5% at lower temperatures.
Cleaning is also aided by detergents possessing hydrophobic (water-hating) molecular chains and hydrophilic (water-loving) components. The use of heat accelerates the dissolving of protein, fats and oils. These can then be removed by the rinse water (6).
Other cleaning agents, these include:
- Organic solvents such as methanol, toluene, acetone, and ethyl acetate
- Aqueous agents, including water, used as a stand-alone cleaning agent or in combination with other substances
- Chelants, which are agents that help to prevent deposition from ionic substances found in scale (like calcium deposits from hard water). Here a chelant is a specialised molecule designed to bind to positively charged metal ions, most commonly calcium and magnesium, in solution. This prevents these ions from forming insoluble precipitates with other ions that may be present. An example is ethylenediaminetetraacetic acid (EDTA)
When selecting cleaning chemicals, their effectiveness is based on the strict control of process parameters including temperature, contact time and the concentration of cleaning agent.

