Our expertise
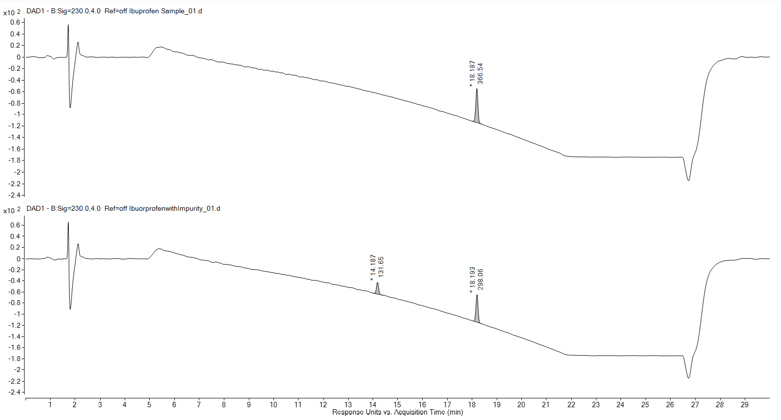
RSSL selected an accurate LC-MS instrument, as the addition of this detector to the client’s UV analytical method provides the ability to analyse an HPLC injected sample by both UV and MS simultaneously and generate highly accurate mass spectrometry data of the observed UV peaks.
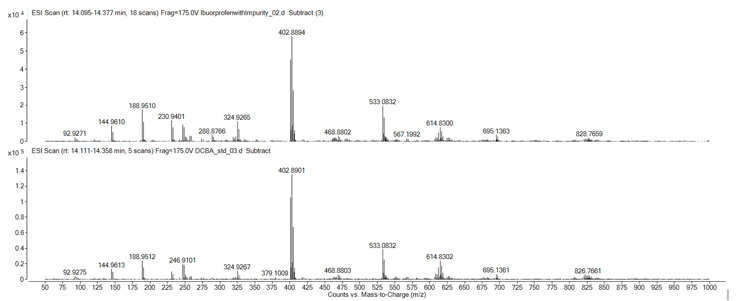
Analysis of the MS data at the retention time of the unknown impurity produced the spectra seen in Figure 2. The mass spectrometry analysis in this instance was performed in negative mode to observe any negatively charged ions. Figure 2 highlighted several mass ions, but careful interpretation determined that mass ion m/z 188.9510 was the deprotonated peak of the impurity compound. Additionally, analysis of the isotopic pattern gave an indicative pattern for the presence of 2 chlorine atoms. The most abundant ion observed was determined to be a sodium adduct dimer ion (2M+Na-2H)-, which further confirmed the impurity had a molecular weight of 189.9583 Daltons.
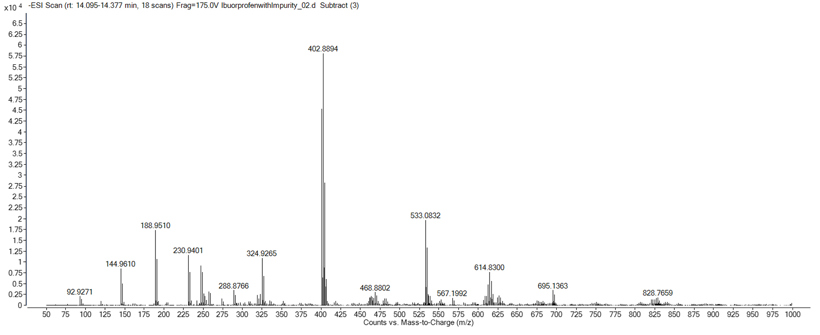
Figure 2: Unknown purity spectra
Outcome
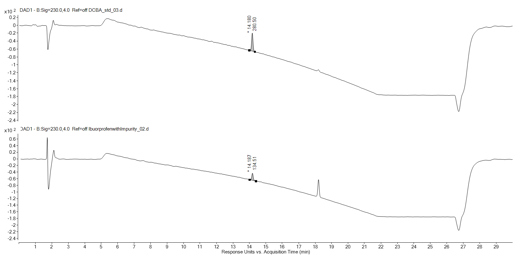
To confirm the identity of the impurity using the mass spectrometry data produced, a standard of DCBA was purchased. This standard was analysed using the same LC-MS method developed to determine the identification of the impurity, which allowed the mass spectra to be directly compared.
Figure 3 shows both the impurity and DCBA mass spectra. The resulting mass spectra displays the same mass ions and a very similar profile, thus confirming the impurity as DCBA. The standard also eluted at the same retention time as the impurity, providing further confirmation (Figure 4).