Design
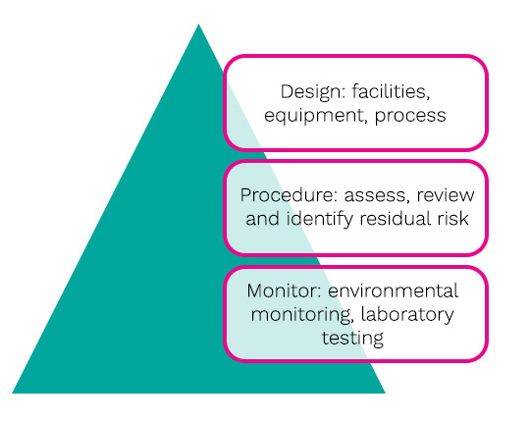
Design should be approached using appropriate, current technologies. with facilities based on suitable people, product and waste flows such as:
- Sterilisation of equipment
- Control of people and gowning
- Materials controls
- The appropriate classification of support and filling rooms
- The use of barrier technology
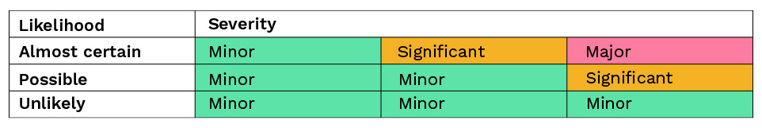
Areas need to be appropriately designed and of sufficient space. Any activities should be supported with equipment of appropriate design, including the implementation of single use, sterile disposable technologies. Procedures need to be well-written as they play an important part of risk mitigation, especially in relation to critical steps such as running the process, line assembly, interventions and cleaning and disinfection. Monitoring needs to be based on a sound, scientifically based strategy. In the context of Annex 1, it is useful to break each clause down in order to assess each point. Figure 4 is an example using Section 8 of the Annex:
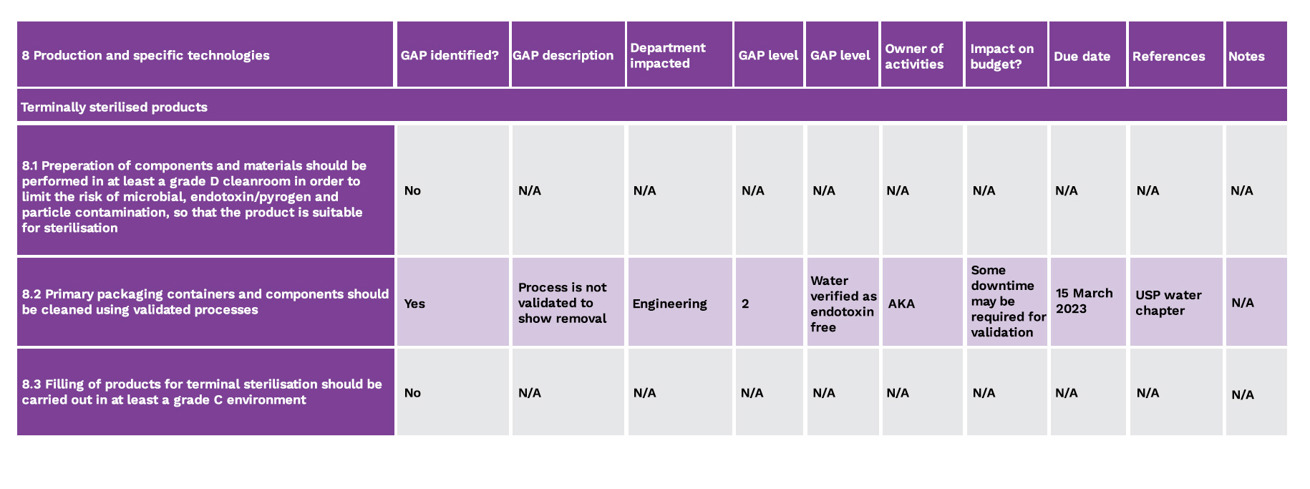
Fig 4 - Example display format for breaking down individual clauses
Once any gaps have been identified they need to be prioritised, depending upon the mitigations in place, how long they need to apply for, and for when any necessary works can be programmed in. Not everything can be done at once and objective criteria is required to sort the gaps into a priority order. This involves risk ranking and risk filtering, estimating likelihoods and consequences.
Putting the analysis together
The analysis forms a core part of the finished document, structured both horizontally and vertically.
With the vertical aspect, each of the main sections within the Annex should be covered. These can be thought of as pillars (detailed in Figure 5)
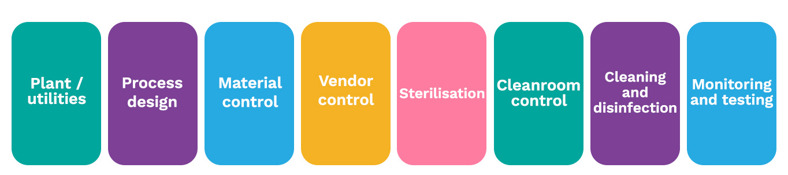
Fig 5 - Vertical pillars of the CSS
With the horizontal aspect, each primary form of contamination should be addressed not only within the pillar, but between pillars, and with consideration of how contamination could be transferred through different activities and across cleanroom grades. For example, this could be represented as: